Taking Synthesis Efficiency to the Next Level

Ammonia Synthesis with LAC™
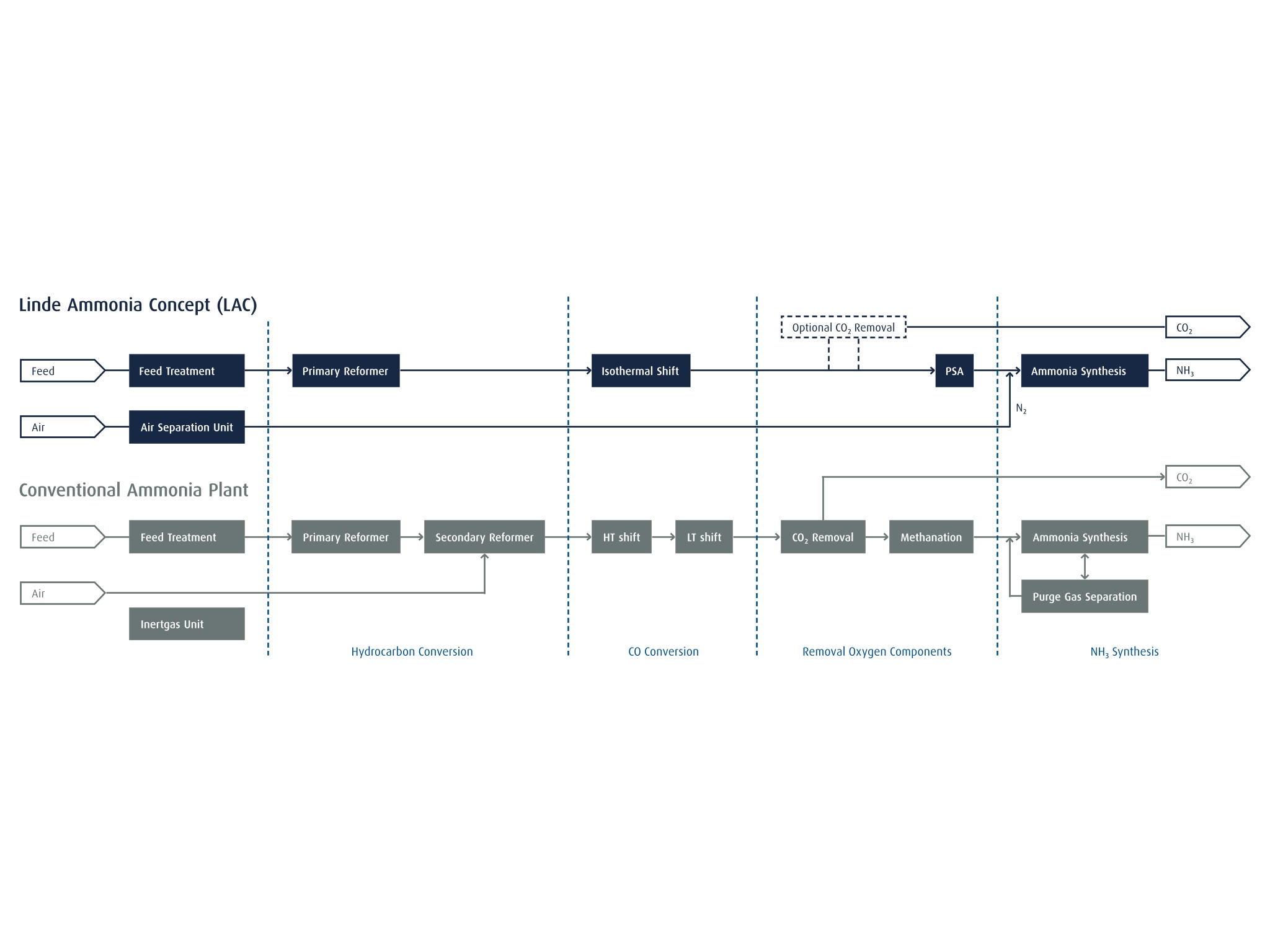
The Linde Ammonia Concept (LAC) is an inert-free process for the production of ammonia (NH3) from natural gas, light hydrocarbons or heavy hydrocarbons. It is based on a combination of proven process steps. An LAC plant primarily comprises a modern hydrogen (H2) plant, a standardized nitrogen (N2) plant (air separation unit or ASU) and a highly efficient NH3 synthesis loop.
Our broad technology portfolio covers all steps in the LAC process chain, from the H2 and air separation plants through pressure swing adsorption (PSA) to carbon capture technologies. We complement our proprietary technologies with a third-party technology license for the ammonia converter to give customers a one-stop offering.
In the H2 plant, the synthesis gas is purified by PSA in a single process step. Pure N2 from the ASU is mixed with the purified H2 at a 1:3 ratio to yield NH3 synthesis gas. Once it has been compressed to between 100 and 200 bar, the syngas is fed into the NH3 synthesis loop, where an exothermic reaction converts it to NH3 at temperatures between 400 and 500°C.
The LAC process simplifies the conventional NH3 path (see graphics below). By replacing air with pure N2, LAC minimizes the accumulation of residual inert impurities such as argon (Ar) and methane (CH4) in the ammonia reactor. This avoids the need for syngas purges and a purge gas separation step. Moreover, the use of inert-free synthesis gas intensifies the NH3 synthesis reaction, which means less catalyst volume is required in the NH3 converter relative to conventional plants. Depending on individual requirements, an optional carbon dioxide (CO2) removal step can be integrated with the LAC process.
The LAC process thus enables CAPEX and OPEX savings while also simplifying plant start-up and operation.
Further specific operating cost efficiencies can be achieved through the sale of valuable LAC by-products. These include H2 and N2 as direct LAC by-products, but also gases such as oxygen (O2), argon (Ar) and carbon dioxide (CO2) provided the plant has been designed to separate these products.


Methanol Synthesis
Methanol (CH3OH, MeOH) is produced from synthesis gas, which in turn is generated by applying steam reforming, partial oxidation, autothermal reforming, dry reforming or a combination of these processes to hydrocarbons.
The synthesis gas is converted into CH3OH in an isothermal reactor. The most commonly used catalyst consists of copper, zinc and aluminum oxides. The catalyst facilitates the chemical reaction between the H2 and carbon monoxide (CO) to form methanol. The reaction takes place at a high pressure (between 50 and 100 atmospheres) and a temperature of around 250°C.
We offer a proprietary isothermal reactor for CH3OH synthesis. This is a fixed-bed reactor cooled by coiled pipes. The catalyst filling is cooled and maintained at an optimum operating temperature through the production of steam in the pipe interiors. The reactor offers the benefits of a pipe reactor but avoids the head storage problems of a straight-pipe reactor.
The catalyst conducts the reaction heat to a cooling pipe bundle embedded in the catalyst embankment, making it possible for the process to operate at optimum temperature. This results in higher performance, lower quantities of catalyst, fewer by-products as well as the efficient recovery of reactor heat with lower reactor costs.